🇺🇸USA Mfg heaters! ⭐️ All parts & materials are made in America– ready to ship 🚛

How To Calculate Heat Loss?
Author: Skip Schaefer
Heat is energy and heat can affect molecules, recall Bill Nye the Science Guy (you dont need to sing along to his music videos). Now everyting is made up of molecules. Air, water, walls, tanks, and metal are all particles where heat can be transferred or lost. There are 3 types of heat transfer: Conduction, Convection & Radiation which I will cover below.
Everything has heat, whether it is hot or cold. It's important to understand the movement of molecules and its relationship to temperature. Warmer water will rise while cooler water will sink because the atoms become closer together adding density. If you heat something up too much, the matter will vibrate so fast they vaporize.
This is why you learn how to calculate heat loss.
Understanding Heat Transfer Types
Now that we have middle school science covered, lets move on to the types of heat transfer or movement. Some might call it heat loss types. Click below for definitions and examples of thermal energy phase changes.

Conduction is the transfer of heat or electricity through a substance by direct contact. The heat transfer rate is dependent upon how much resistance exists between objects of differing temperatures. (That's insulation) A good example of conduction is placing a spoon in a glass of hot coffee. The metal spoon warms up because it touched the hot molecules. The coffee solution actually had heat loss.
Now not all objects are good conductors of heat. Metal is a good conductor, so it heats up fast in hot coffee. Glass is a poor conductor. The outside of the glass is cooler than the metal spoon, even though they are both touching hot coffee.
Whether a substance acts as a thermal conductor or insulator depends on the thermal resistive properties of the matter. Thermal resistance (R) is a measure of an object's ability to decrease heat transfer by way of conduction through a given thickness of the substance. Mathematically, R is: Calculating Heat Loss from Industrial Systems to determine heat tracing where L is the insulation thickness in inches, k is thermal conductivity, (BTU)(in)/(ft2)(oF)(hr) As the thickness (L) changes, it affects the R value, or thermal resistance of an insulation. K values are constants that are specific to the physical properties of a given material. They measure thermal conductivity. Here is the Conduction Formula
When \(a \ne 0\), there are two solutions to \(ax^2 + bx + c = 0\) and they are $$x = {-b \pm \sqrt{b^2-4ac} \over 2a}.$$ $$ R = {L \over k}$$

Convection is the transfer of heat through fluids (gases or liquids) from a warmer spot to a cooler spot. Place an ice cube in hot coffee and the molecules on top become cooler and dense therefore moving to the bottom.
If you heated the bottom of the coffee mug the opposite would happen. The more dense liquid on bottom would expand becoming lighter and would move to the top. This movement of heat is called convection.
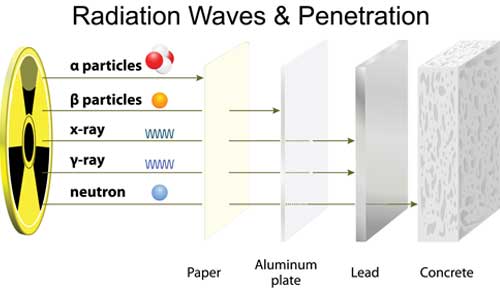
Radiation is the transition of energy (heat) from one body in the form of waves or particles.
Sounds simple but radiation can come in different forms and energy strengths, here are some examples of radiation:
- • ultraviolet light from the sun
- • heat from a camp fire
- • light from a candle
- • x-rays from an x-ray device
- • alpha particles emitted from radioactive decay of uranium
- • cooking waves from a microwave oven
- • electromagnetic radiation from your mobile phone
Heat Loss Formulas & Calculations
In most low-to-medium temperature applications, radiation and convection account for about 10% of the heat loss of objects. The formula for calculating heat loss of a system can be calculated by adding in 10%.
Calculating Flat Surface Heat Loss
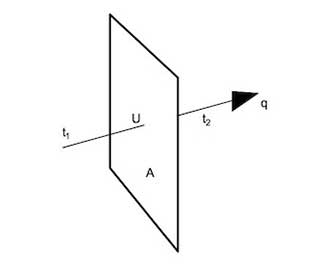
The term “heat loss” commonly refers to the thermal transfer of an object to its ambient environment. For example a wall is at a temperature above the ambient temperature. The formula for calculating heat loss of a system through conduction (Fourier's Law), expressed in BTU/hour is:
$$ Q = {(U)(A)(ΔT)}$$
Where U is the thermal conductance, BTU/(ft2)(Fº)(hr)
A is the surface area of object, ft2
ΔT is the temperature difference (T1 -T2), Fº
Conductance is the inverse of resistance, R, and can be expressed as U = 1/R So, another equation for general heat loss in BTU/hr is:
$$ Q = {k(A)(ΔT)(1.1) \over L} $$
How Is Heat Loss Measured?
Heat loss is measured in kWs or BTUs. It is a function of heat transfer rates. Heat transfer rates in walls, floors and roofs are measured in U values. To calculate the U value of a wall, pipe, metal or water tank, you need to know the composition and thickness of each part.
Electricity is normally sold by kilowatt hours, but the equations above are BTU/hr. Just convert BTU to watts, where 1 watt equals 3.412142 BTU and multiply times (L). The answer will be in W/hr.
$$ Q = {k(A)(ΔT)(1.1) \over (L)(3.412142)} $$
Heat Transfer Rate Equation
Conductive heat transfer can be expressed with:
q = (k / s) A dT
= U A dT (1)
where
q = heat transfer (W, J/s, Btu/hr)
k = Thermal Conductivity of material (W/m K or W/m Cº, Btu/(hr Fº ft2/ft))
s = material thickness (m, ft)
A = heat transfer area (m2, ft2)
U = k / s
= Coefficient of Heat Transfer (W/(m2K), Btu/(ft2 h Fº)
dT = t1 - t2= temperature gradient - difference - over the material (Fº)
Heat Loss From a Water Tank Calculator

Open water tanks like plating tanks, di water tanks, hot tubs or swimming pools lose excessive heat due to evaporation. SAVINGS TIP: Cover open water containers with insulation when not in use. The overall heat loss from an open water tank can be expressed as:
Q = Qevaporation fluid + Qradiation fluid + Qtransmission through walls
The heat loss due to evaporation of water from a surface of an open tank is dominant at higher water temperatures. Any heat loss through insulated walls is minimal, this is especially try for polypro tanks. Polypropelne is an excellent insulator.
Remember– heat rises, so all the energy is being released on the top.
| Water Temperature (Fº) | Heat Loss from Liquid Surface (Btu/ft2hr) | Heat Loss Through Tank Walls (Btu/ft2hr) | |||||
|---|---|---|---|---|---|---|---|
| Evaporation Loss | Radiation Loss | Total | Bare Steel Uninsulated | Insulated (inches) | |||
| 1 | 2 | 3 | |||||
| 90 | 80 | 50 | 130 | 50 | 12 | 5 | 4 |
| 100 | 160 | 70 | 230 | 70 | 15 | 8 | 6 |
| 110 | 240 | 90 | 330 | 90 | 19 | 10 | 7 |
| 120 | 360 | 110 | 470 | 110 | 23 | 12 | 9 |
| 130 | 480 | 135 | 615 | 135 | 27 | 14 | 10 |
| 140 | 660 | 160 | 820 | 160 | 31 | 16 | 12 |
| 150 | 860 | 180 | 1040 | 180 | 34 | 18 | 13 |
| 160 | 1100 | 210 | 1310 | 210 | 38 | 21 | 15 |
| 170 | 1380 | 235 | 1615 | 235 | 42 | 23 | 16 |
| 180 | 1740 | 260 | 2000 | 260 | 46 | 25 | 17 |
| 190 | 2160 | 290 | 2450 | 290 | 50 | 27 | 19 |
| 200 | 2680 | 320 | 3000 | 320 | 53 | 29 | 20 |
| 210 | 3240 | 360 | 3600 | 360 | 57 | 31 | 22 |
- the values above are for 60 Fº still ambient air
Example Formula Heat Loss From Open Water Tank
Here is a sample calculation of a water tank heat loss using the Table above (NOTE: the room temperature is at 60º F), where:
- Water temperature: 150 Fº
- Surface area: 10 ft2 (You calculate this by measuring the top open area; IE 4' x 2.5'=10 ft 2
- Un-insulated steel tank walls area: 50 ft2 (Add up each area on a metal rectangle container; 10 ft 2 + 15 ft 2+ 10 ft 2+15 ft 2= 50 ft 2 )
Q(evaporation fluid + radiation fluid) = ( 1040 Btu/(ft 2 hr)) (10 ft2)
= 10400 Btu/hr
Qtransmission through walls = ( 180 Btu/(ft Btu/(ft2 hr)) (50 ft2)
= 9000 Btu/hr
Q = Q(evaporation fluid + radiation fluid) + Qtransmission through walls
= (10400 Btu/hr) + (9000 Btu/hr)
= 19400 Btu/hr
Need More Help?
When you become a customer, Skip Schaefer will put his almost 50 years of experience, designing heating processes, to work for you. His electric heaters are used by manufacturers worldwide. IHS offers a wide range of thermal products, including deep and large plating tank heaters. If you have a special manufacturing problem, Skip can solve it, talk to him today.



